Pure water!
For optimizing water used in air humidification, two methods have proved particularly good: water purification by softening and reverse osmosis. Both processes are briefly presented below and their combined effects described. Water softening and reverse osmosis are two different water purification processes, which can complement one another.
Softening
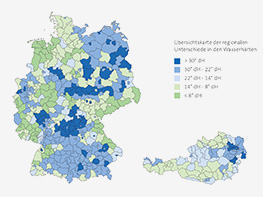
Calcium and magnesium make water hard and result in deposits in technical systems. In softening plants, these substances are continuously exchanged for sodium and are therefore automatically eliminated from the water. This causes the water to become soft and the systems are protected against calcification and corrosion — this is the basis of hygienic operation. As a result, the Condair reverse osmosis system operates more efficiently.
Natural water transport
The technical process of reverse osmosis runs according to the natural principles of water transport in plants. Plant cells are surrounded by an osmotic membrane, which is permeable to pure water but not to molecules dissolved in the water. Outside and inside the cells there are large molecules of different concentrations. Physical forces then attempt to balance the concentrations on both sides of the membran. Water therefore migrates by osmosis through the membrane to the side with the greater molecule concentration, in order to dilute it. This is the reason why, for example, ripe, sweet cherries and tomatoes burst after rainfall. The pure rainwater on the fruit migrates by osmosis through the skin, which is a natural membrane, to the sugar molecules inside the fruit (Figure 2). Therefore, excess pressure gradually builds up in the fruit until the skin bursts.
Osmosis reversed
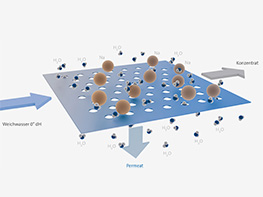
Technically, the principles of naturally occurring osmosis are reversed, for example, to purify water. Contaminated water is forced through an artificial membrane from the side with the higher molecule concentration to the side with the pure water. After the membrane, the pure water is collected as permeate and can be used as hygienic humidification water. Molecules and salts that concentrate in front of the membrane, so-called concentrate, are continuously flushed away. Depending on the quantity, use and salt content of the untreated water, the pump, which presses the impure water against the membrane, must be capable of generating pressures of up to 100 bar. In order to withstand the pump pressure, the membrane is drawn over an appropriately pressure-resistant car-rier material. In most cases, the membranes are wound to produce strong modules and are pro-tected by a tube. Originally, reverse osmosis (Figure 3) was developed in order to obtain low-salt drinking water from sea water (‘contaminated’ with salt). In the meantime, this method has also been used for other types of water treatment or for dialysis. For example, concentrated juice or al-cohol-free beer is produced by this method. With the help of reverse osmosis, not only can alcohol, sugar and salt be removed from water, but also viruses, bacteria and proteins. For hygiene reasons, the high-quality permeate produced by Condair’s ‘AT2’ reverse osmosis is made available in a stainless-steel pressure accumulator that is sealed off from the atmosphere (Figure 4).
The key element of any reverse osmosis process is a membrane, which channels off the large molecules with the concentrate. The pure water (permeate) can now be used to humidify the air hygienically.
Water purification for air humidification systems - Andreas Zwigart, Condair GmbH
Natural water transport
The technical process of reverse osmosis runs according to the natural principles of water transport in plants. Plant cells are surrounded by an osmotic membrane, which is permeable to pure water but not to molecules dissolved in the water. Outside and inside the cells there are large molecules of different concentrations. Physical forces then attempt to balance the concentrations on both sides of the membran. Water therefore migrates by osmosis through the membrane to the side with the greater molecule concentration, in order to dilute it. This is the reason why, for example, ripe, sweet cherries and tomatoes burst after rainfall. The pure rainwater on the fruit migrates by osmosis through the skin, which is a natural membrane, to the sugar molecules inside the fruit (Figure 2). Therefore, excess pressure gradually builds up in the fruit until the skin bursts.
Osmosis reversed
Technically, the principles of naturally occurring osmosis are reversed, for example, to purify water. Contaminated water is forced through an artificial membrane from the side with the higher molecule concentration to the side with the pure water. After the membrane, the pure water is collected as permeate and can be used as hygienic humidification water. Molecules and salts that concentrate in front of the membrane, so-called concentrate, are continuously flushed away. Depending on the quantity, use and salt content of the untreated water, the pump, which presses the impure water against the membrane, must be capable of generating pressures of up to 100 bar. In order to withstand the pump pressure, the membrane is drawn over an appropriately pressure-resistant car-rier material. In most cases, the membranes are wound to produce strong modules and are pro-tected by a tube. Originally, reverse osmosis (Figure 3) was developed in order to obtain low-salt drinking water from sea water (‘contaminated’ with salt). In the meantime, this method has also been used for other types of water treatment or for dialysis. For example, concentrated juice or al-cohol-free beer is produced by this method. With the help of reverse osmosis, not only can alcohol, sugar and salt be removed from water, but also viruses, bacteria and proteins. For hygiene reasons, the high-quality permeate produced by Condair’s ‘AT2’ reverse osmosis is made available in a stainless-steel pressure accumulator that is sealed off from the atmosphere (Figure 4).
The key element of any reverse osmosis process is a membrane, which channels off the large molecules with the concentrate. The pure water (permeate) can now be used to humidify the air hygienically.
Water purification for air humidification systems - Andreas Zwigart, Condair GmbH